¶ Introduction
This document is meant to help prove certain designs are able to resist extreme temperatures (-40 °C to RT) unlike the ones that will be experienced during the flight.
¶ Definitions and Abbreviations
- RT: Room temperature
- ABV : Abbreviations
¶ Relevant Knowledge Needed
Dry Ice is the solid for of carbon Dioxide (CO2). It is around 78.5 °C, is non-flammable, and sublimates below this temperature (it does not melt, but turns directly into gas).
It can be dangerous if not handled with caution, it is imperative to follow the following instructions
¶ Safety procedure
- Use gloves when handling dry ice (temperature is -78.5 °C )
- Don’t keep dry ice in a confined area with no air flow as the dry ice will sublimate
- Make holes in styrofoam box as dry ice will sublimate and cause a lot of internal pressure if the box is sealed
- If conserving dry ice in a fridge or other confined space, when opening it, let the air flow for a few seconds before going inside or in front
To determine the amount of dry ice requires to be able to achieve a thermal equilibrium of -40°C, certain calculations are necessary.
¶ Calculations
Heat required to cool the component and surrounding air
- Q= mcomponentcmaterial∆T + maircair∆T
Where :
c = specific heat capacity
note: the mass of the air can be calculated with the volume of the box and the density of air
Density of air at room temperature : 1.204 kg/m3
Latent heat of sublimation of dry ice: L=571’000 J/kg
Specific heat capacity of air: cair=1870 J/kg/K
The heat of the sublimation has to be equal to the heat required to cool down the component and the air
Calculation
- Q=M dry ice L
- M=Q/L=mc∆T/L
With :
M= mass of dry ice
Add a 20% margin for the mass to account for the heat loss and adjust depending on the temperature of the component
Example: to cool a 70g steel line cutter (without accounting for the cooling of the air surrounding it)
c = 420 J/(kg x K)
Tf = -40°C =233 K
Ti = 20°C = 293 K
M = (70 x 10^(-3)* 420 * 60)/571000=3.08 g
Adding a 20% safety:
M=3.70 g
¶ Design Options
Because of the extreme low temperature of dry ice, it can be a very efficient way of creating a low temperature environment.
¶ General procedure:
- Place the pre-calculated required amount of dry ice in the styrofoam box
- Place component to be tested
- wait until thermal equilibrium is reached
- Measure the temperature of the box and adjust the amount of dry ice depending on the result
- Test the component
Example of a diagram for dry ice usage for the line cutter low temperature experiment:
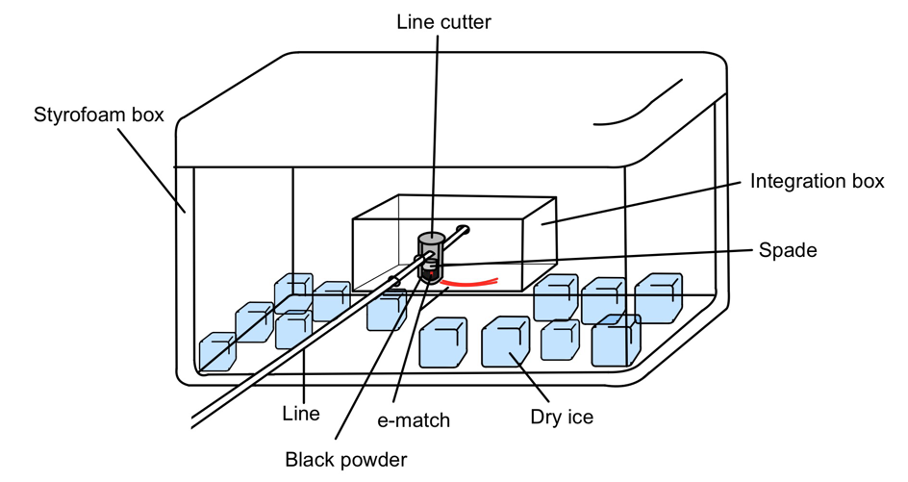
Easily accessible and free at EPFL (free access)
Easy to use
Efficient
Can be dangerous if not handled properly
Liquid Nitrogen can be used in a similar way as dry ice and is also extremely effective in cooling down components due to its extreme temperature.
¶ General procedure:
- Place liquid nitrogen in a shallow dish
- Place component to be tested
- wait until thermal equilibrium is reached
- Measure the temperature of the box and adjust the amount of liquid nitrogen depending on the result
- Test the component
Very stable over time
Precise
Non-flammable
Expensive
Not easily accessible
Relatively dangerous (more so than dry ice)
Climate chambers are designed to maintain specific humidity and temperature levels for testing and research. As such they would be perfect for such an experiment however they cannot be taken into consideration as they are extremely expensive, and inaccessible.
Precise
Easy
Far too expensive
¶ Narrowing the Design Options
As said previously, a climate chamber would be ideal but cannot be taken into account because of cost, hence we have to choose between the remaining two options.
¶ Requirements and Design Criterias
In general, both remaining methods have to fulfill different requirements.
Here are the main criterias for the selection:
| Cost | Precision | Stability | Rapidity of setup |
|
| The cost of the testing setup in itself | How precise do we need the relative humidity percentage to be | What amount of variation of the relative humidity percentage over time can we allow| How quickly does the experiment need to be prepared |
¶ For this experiment, the applicable criterias for the selection are:
These criterias take into account the fact dry ice is in free access at EPFL (refer to the relevant documents at the end of the document for more detail)
Dry Ice:
- More cost effective (free)
- Easier to set up
- Easily accessible (free access)
Liquid Nitrogen
- More precise
- Stable over time
¶ Trade-off Results
For most of the required low temperature tests in Recovery, cost, access and ease of use are far more important than stability over time as the tests will be done relatively quickly and not over long periods of time. As for precision, although liquid nitrogen is more precise, Dry ice is precise enough for the tests being done at this time.
¶ Detailed Design
REMOVE THIS TEXT
How did you interate on the design?
What were you trying to optimise?
Why did you stop iterating?
TO COMPLETE WHILE/AFTER TESTING
REMOVE THIS TEXT
¶ Iterations
REMOVE THIS TEXT
Description of the version.
REMOVE THIS TEXT
Main Advantages
Main Disadvantages

REMOVE THIS TEXT
Description of the version.
REMOVE THIS TEXT
Main Advantages
Main Disadvantages

REMOVE THIS TEXT
Description of the version.
REMOVE THIS TEXT
Main Advantages
Main Disadvantages
¶ Test Reports
REMOVE THIS TEXT
If you used tests to itirate link the test reports here.
REMOVE THIS TEXT
- 2024_C_SS_CAR-TEST_TRP: The AV shall trigger and power the recovery events.
¶ Relevant Documents
- EPFL dry ice free access Accessibility of dry Ice at EPFL
Dry ice is accessible in free access 24/7 in the room SV 0409
- 2024_C_SE_RE_REQ_16 Temperature range
The LV shall at least operate within the temperature range from [-60]°C to [+70]°C for SRAD components and from [-40]°C to [+50]°C for COTS components.
- 2024_C_SE_REQ_01 LV declaration of purpose
The LV shall be a mean of testing spaceshot technologies and participate in the L9 flight category at the 2025 EuRoC competition ([9000]m apogee).